INOPP Forum
Australian Public Assessment Report (AusPAR)
Quote from VigiServe Admin on March 23, 2021, 7:01 AMThe Therapeutic Goods Administration (TGA) evaluates applications for the registration of prescription medicines for entry on the Australian Register of Therapeutic Goods (ARTG).
The Australian Public Assessment Report (AusPAR) includes information on the outcome of the evaluation process and the rationale for the decision to register or to reject registration. The AusPAR is published on the TGA website. The publication of an AusPAR is an important part of the transparency of the TGA's decision-making processes.
The TGA currently publishes an AusPAR for the majority of applications under the category of "major submission" for the inclusion of a prescription medicine on the ARTG. This includes submissions for new chemical and biological entities, extension of indication/s, and significant variations to already registered prescription medicines. More details on the submission types in relation to which AusPARs are published are listed under Section 4.1 - AusPARs by application type.
The AusPAR is a static document that is submission specific. It records TGA's consideration of a submission for that prescription medicine at a specific point in time of the regulatory process. The AusPAR is not intended to be updated to reflect variations to a prescription medicine after it has been registered. A new AusPAR will be published where an application is subsequently made for an additional indication and may be published where an application is later made for major variation.
The first AusPAR was published in November 2009 as part of TGA's implementation of the increased transparency strategy under the Business Process Reforms for Prescription Medicines. The TGA's approach to AusPARs is consistent with similar transparency measures introduced within the European Union and United States.
AusPARs published by the TGA are located at Australian Public Assessment Reports for prescription medicines (AusPARs).
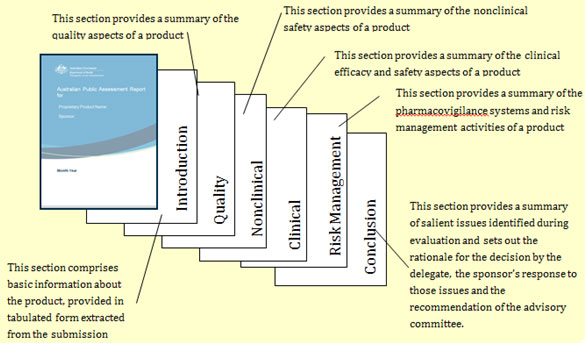
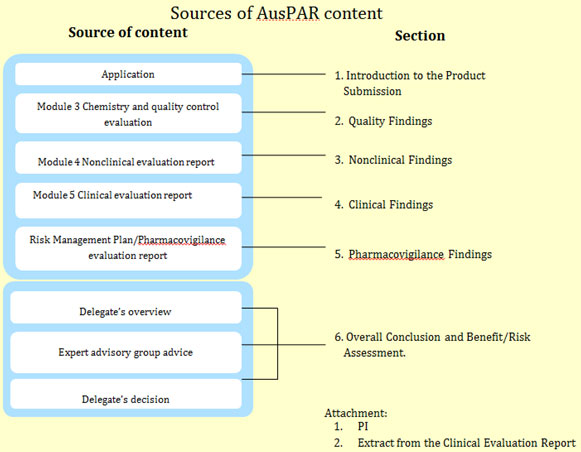
Typical AusPAR components
The Therapeutic Goods Administration (TGA) evaluates applications for the registration of prescription medicines for entry on the Australian Register of Therapeutic Goods (ARTG).
The Australian Public Assessment Report (AusPAR) includes information on the outcome of the evaluation process and the rationale for the decision to register or to reject registration. The AusPAR is published on the TGA website. The publication of an AusPAR is an important part of the transparency of the TGA's decision-making processes.
The TGA currently publishes an AusPAR for the majority of applications under the category of "major submission" for the inclusion of a prescription medicine on the ARTG. This includes submissions for new chemical and biological entities, extension of indication/s, and significant variations to already registered prescription medicines. More details on the submission types in relation to which AusPARs are published are listed under Section 4.1 - AusPARs by application type.
The AusPAR is a static document that is submission specific. It records TGA's consideration of a submission for that prescription medicine at a specific point in time of the regulatory process. The AusPAR is not intended to be updated to reflect variations to a prescription medicine after it has been registered. A new AusPAR will be published where an application is subsequently made for an additional indication and may be published where an application is later made for major variation.
The first AusPAR was published in November 2009 as part of TGA's implementation of the increased transparency strategy under the Business Process Reforms for Prescription Medicines. The TGA's approach to AusPARs is consistent with similar transparency measures introduced within the European Union and United States.
AusPARs published by the TGA are located at Australian Public Assessment Reports for prescription medicines (AusPARs).
Typical AusPAR components
- You need to login to have access to uploads.